GASROS
The GASROS study aims to discover genetic factors that contribute to stroke risk and outcome. Genetic variance in a population can be examined by looking at genetic polymorphisms. By collecting medical history and blood samples from AIS and TIA patients, this study examines the genetic polymorphisms in those who present with cerebrovascular disease and specific stroke-associated phenotypic traits. By additionally collecting follow up information at 3 and 6 months after stroke, this data can be assessed and analyzed to compare patient’s genetic markers with their outcome after stroke. With the aim to develop early preventative measures to treat and reduce or prevent stroke, this study strives to identify specific genetic clues as targets for developing new treatments and provide personalized medicine.
Importance of location
GASROS has a wealth of information, including manual identification of which areas in the brain are affected by white matter hyperintensities or stroke. This allowed us to investigate the spatial contribution of lesions in the brain, be that the acute lesion, i.e. the stroke, or the chronic leasion, i.e. the white matter hyperintensities.
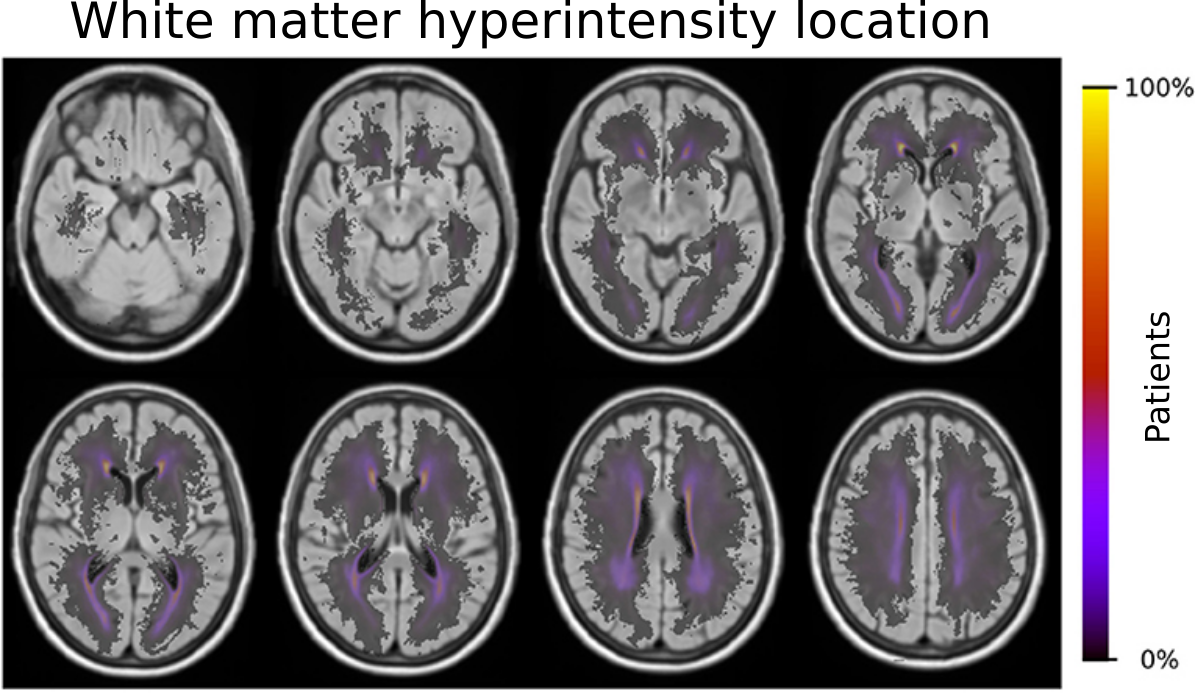
With the outlines of the white matter hyperintensities, and the creation of a template to differentiate regions in the brain based on their blood supply by major cerebral arteries, we were able to identify risk factors which contribute to an increase of disease burden in these areas. In brief, older age, male sex, small-vessel stroke subtype, hypertension, hyperlipidemia, and smoking demonstrated effects that shifted the prevalence of the disease burden in these territories. A more complete summary can be found in our coverage by Advances in Motion or the corresponding paper in Frontiers in Neurology. Importantly, as part of our study, we created a template of these territories. We made this template publicly available (see our Data section) to help other investigators execute large scale studies and further disentangle the importance of location and contribution factors to spatially specific effects in stroke and beyond.
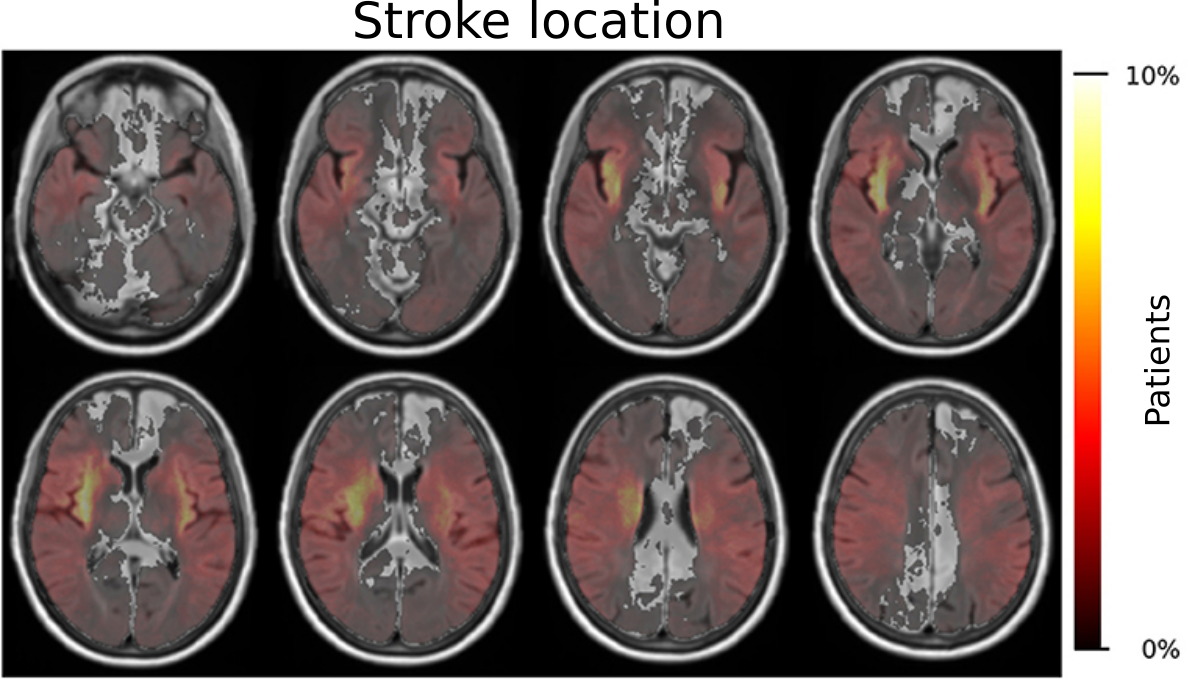
Additionally, we further investigated the importance of stroke location with respect to patients outcome adding to the growing opinion that the stroke volume alone, i.e. how big the by the stroke affected area in the brain is, does not fully capture this disease. Within GASROS, we investigated this approach in multiple ways. First, we independently identified regions of the brain that most significantly contributed to a worsening of the outcome by using voxel-based lesion symptom mapping. In this study, we, e.g., identified that injury to the white matter, post-central gyrus, putamen, and perculum were implicated with poor outcome, as measuerd by the modified Rankin Scale. The full investigation is openly available in our accompanying publication "The role of acute lesion topography in initial ischemic stroke severity and long-term functional outcomes".
Additionally, by incorporating structural connectivity information from healthy participants, we demonstrated that specific areas in the brain that are important for efficient information transport, have a much higher contribution to the patients outcome. In the field of connectomics (brain connecitivy analysis), these regions are referred to as the rich-club. With the help of the GASROS study, we were able to create an automated assessment if these regions were affected, to create a translational biomarker of stroke outcome, which can readily be assessed using standard clinical imaging, as it is often used in the emergency room. A full description of this work is openly available in the corresponding publication Rich-Club Organization: An Important Determinant of Functional Outcome After Acute Ischemic Stroke. Moreover, with the help of the SALVO study, we were able to expand this concept, thereby significantly improving our understanding of how structural and functional connectivity in the brain play a key role for stroke patients.
Understanding post-stroke outcome
Besides the location of the acute and chronic lesions in acute stroke cohorts, there are other contributing biological factors that we can quantify based on the data from the acute imaging protocol in the emergency room. The ultimate goal is to understand and model stroke outcome at the time of admission, which can then further help stratify patient population, helping to optimize treatment options and developing new approach addressing the individual contributing factors. While we investigated many aspects of these contributing factors, we will outline two more recent areas of investigations in our lab, specifically, ventricles and effective reserve.
Ventricles
The ventricular system is one of the most promenent features visible on an MRI examination, due to their central location and there relatively large size. In general, ventricular volume increases with age, however, in certain diseases, pathological expansion has been observed. Nonetheless, in stroke patients, the ventricles as a potential biomarker, remain largely unexplored.
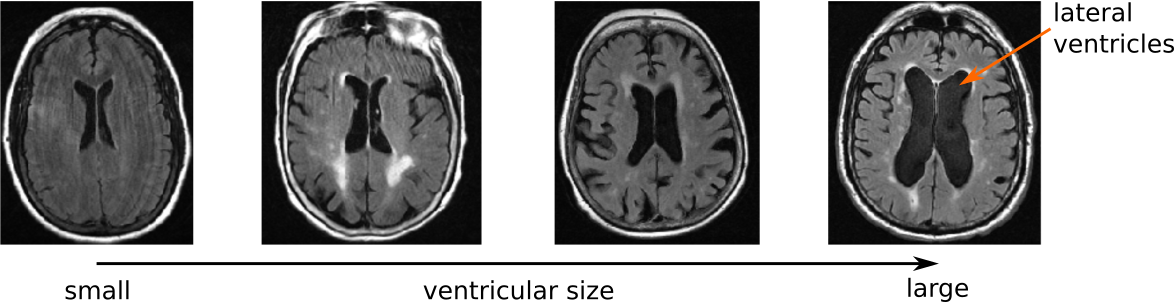
Utilizing the GASROS study, we first developed an automated segmentation tool for the ventricular system in clincal scans. This helped us in two independent manners. First, we analyzed the shape of the ventricles to see if clinical aspects of stroke patients, such as hypertension, white matter hyperintensity volume, and outcome, are associated with specific shape differences. Using advanced image analysis methodology (conditional joint models of shape, image features, and clinical indicators), we were able to create a model of the ventricular shape and explore the individual contributions. This was first published as part of the Medical Image Computation and Computer Assisted Intervention (MICCAI) international conference (Patient-specific Conditional Joint Models of Shape, Image Features and Clinical Indicators), and is illustrated in the video below.
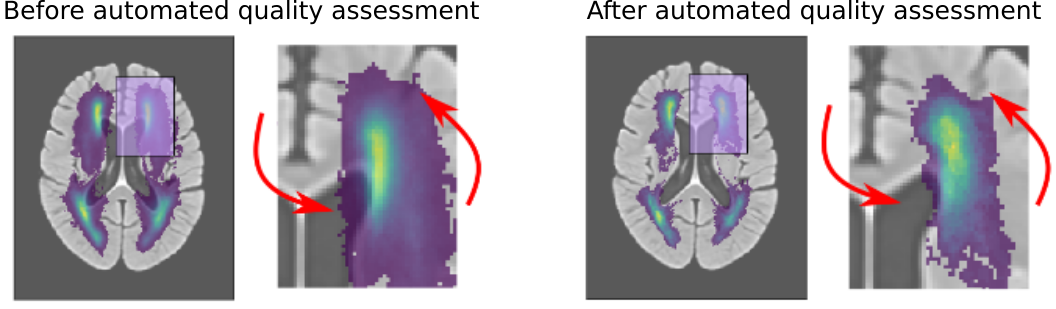
This ventricle segmentation also gave us an important advantage for evaluating automated processing pipelines. As described in the section above, location plays an important role in functional stroke outcome. However, to evaluate the location in large data sets, an image registration step is required, which aligns the patient's MR scan to a template. This is a particularly difficult step, when working with clinical data that, due to medical time constraints in treatment, are acquired at a lower resolution. While the registration can still be performed, it is important to assess if and how much the computer struggled with the alignment. Utilizing the ventricle segmentation, however, we were able to automatically assess the accuracy of this alignment, in addition to employ age-specific templates as an intermediate step, which can significantly reduced the error rate in the registration. Importantly, through this methodology, we were able to move from the typical qualitative to a quantiative assessment strategy of automated processing pipelines. The figure below demonstrates the improvement after identifying errors in the alignment using our quantiative, automated approach, when creating white matter hyperintensity distributions on a template. For more details, please see the corresponding publication on "Automated Image Registration Quality Assessment Utilizing Deep-learning based Ventricle Extraction in Clinical Data".
Effective reserve
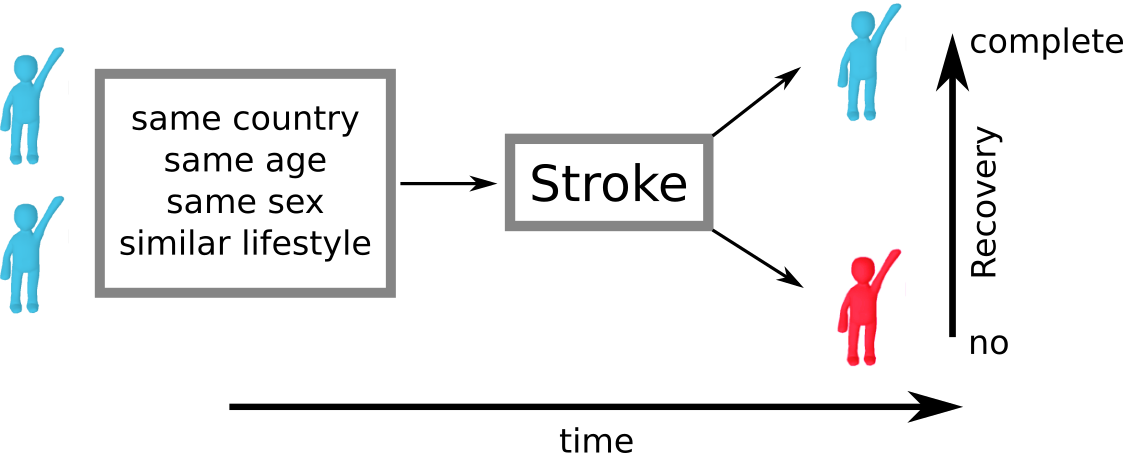
Despite all the efforts in determining the contributing factors, a key challenge remains. Assume we look at two patients from the same country, same age, same sex, and similar lifestyles, who also have a stroke in roughly the same area of the brain. In clinical practice, their outcome can still be vastly different. This suggests that an underlying mechanism exists, which influences patient recovery, but which we are unable to identify and measure at this point: effective reserve. Effective reserve is an extension of the principle of brain reserve, which aims to describe the brain's capacity to compensate for negative impacts of pathology. A simple metaphor for reserve represents a bucket that holds back the effects of pathological processes (water). At some point, this bucket is filled to it's maximum and cannot compensate for negative effects anymore; when the bucket overflows, symptoms manifest.
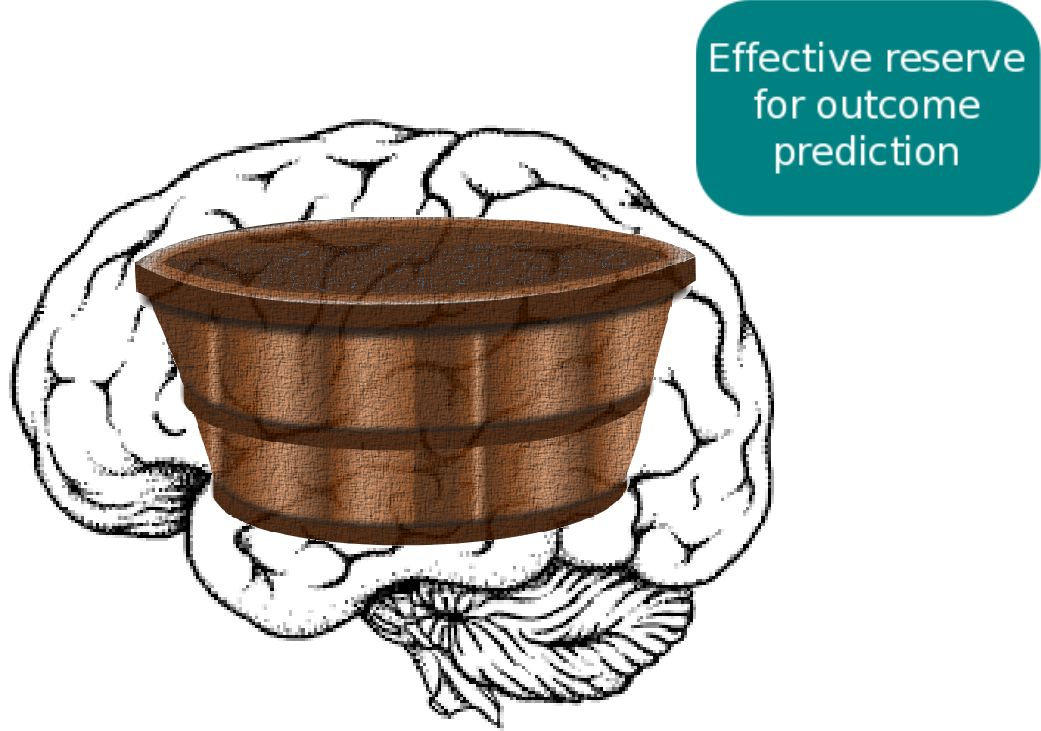
In general, scientists differentiate between two types of reserve. Brain reserve considers the structural or biological compensation mechanism, where as cognitive reserve describes the mechanism originating from functional and/or cognitive activity. However, these principles do not take existing pathology into account. If a brain's reserve is already compensating for existing pathology, a new event, such as a stroke, cannot utilize this 'occupied' reserve. The remaining, available reserve is then called effective reserve.
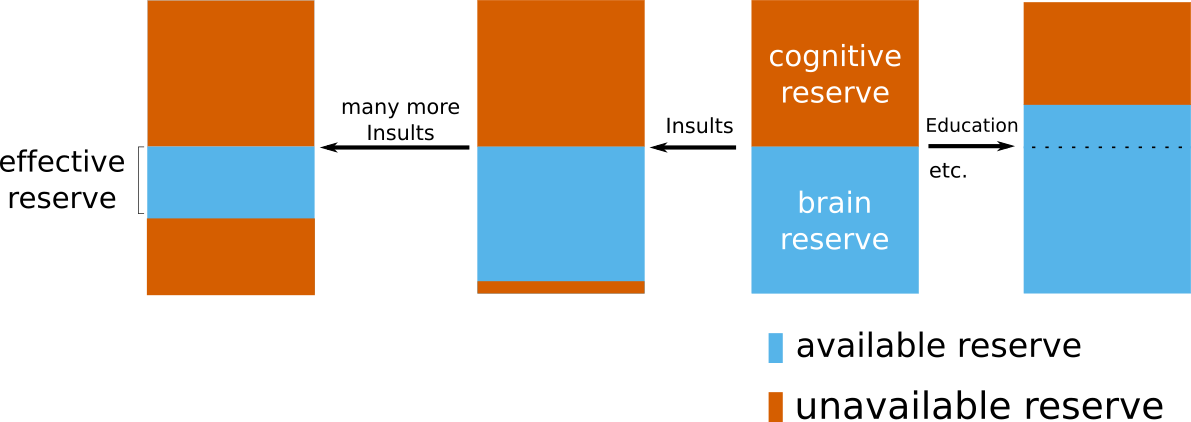
With the help of the GASROS study, we were able to investigate this concept in a stroke population. The full study is described in our journal publication "Effective reserve: a latent variable to improve outcome prediction in stroke", or in a high-level summary of our results here. In brief, we were able to model effective reserve and demonstrate that an increase in this capacity to compensate for negative effects is directly related with a better post-stroke outcome. A possible biological explanation of what effective reserve in stroke patients may represent, is vascular health. With the available additional information on hypertension in our patients, which can be seen as a proxy of vascular health, we demonstrated that non-hypertensive patients exhibit a higher effective reserve, i.e. a higher capacity to compensate for negative effects. While further studies are necessary to fully elucidate the building blocks of this concept, our study indicates that we are starting to uncover a crucial element of functional post-stroke outcome.
Other findings as a direct result of the GASROS study
Etherton MR, Wu O, Cougo P, Giese AK, Cloonan L, Fitzpatrick KM, Kanakis AS, Boulouis G, Karadeli HH, Lauer A, Rosand J, Furie KL, Rost NS.
Neurology. 2017 May 2;88(18):1701-1708. doi: 10.1212/WNL.0000000000003890. Epub 2017 Apr 5.
Genetic Risk Prediction of Atrial Fibrillation.
Lubitz SA, Yin X, Lin HJ, Kolek M, Smith JG, Trompet S, Rienstra M, Rost NS, Teixeira PL, Almgren P, Anderson CD, Chen LY, Engström G, Ford I, Furie KL, Guo X, Larson MG, Lunetta KL, Macfarlane PW, Psaty BM, Soliman EZ, Sotoodehnia N, Stott DJ, Taylor KD, Weng LC, Yao J, Geelhoed B, Verweij N, Siland JE, Kathiresan S, Roselli C, Roden DM, van der Harst P, Darbar D, Jukema JW, Melander O, Rosand J, Rotter JI, Heckbert SR, Ellinor PT, Alonso A, Benjamin EJ; AFGen Consortium.
Circulation. 2017 Apr 4;135(14):1311-1320. doi: 10.1161/CIRCULATIONAHA.116.024143. Epub 2016 Oct 28.
Antecedent Aspirin Use Is Associated with Less Severe Symptoms on Admission for Ischemic Stroke.
Nelson S, Cloonan L, Kanakis AS, Fitzpatrick KM, Shideler KI, Perilla AS, Furie KL, Rost NS.
J Stroke Cerebrovasc Dis. 2016 Oct;25(10):2519-25. doi: 10.1016/j.jstrokecerebrovasdis.2016.06.032. Epub 2016 Jul 18.
Loci associated with ischaemic stroke and its subtypes (SiGN): a genome-wide association study.
NINDS Stroke Genetics Network (SiGN); International Stroke Genetics Consortium (ISGC).
Lancet Neurol. 2016 Feb;15(2):174-184. doi: 10.1016/S1474-4422(15)00338-5. Epub 2015 Dec 19.
Genome-wide meta-analysis of cerebral white matter hyperintensities in patients with stroke.
Traylor M, Zhang CR, Adib-Samii P, Devan WJ, Parsons OE, Lanfranconi S, Gregory S, Cloonan L, Falcone GJ, Radmanesh F, Fitzpatrick K, Kanakis A, Barrick TR, Moynihan B, Lewis CM, Boncoraglio GB, Lemmens R, Thijs V, Sudlow C, Wardlaw J, Rothwell PM, Meschia JF, Worrall BB, Levi C, Bevan S, Furie KL, Dichgans M, Rosand J, Markus HS, Rost N; International Stroke Genetics Consortium.
Neurology. 2016 Jan 12;86(2):146-53. doi: 10.1212/WNL.0000000000002263. Epub 2015 Dec 16.
Integrative Mouse and Human Studies Implicate ANGPT1 and ZBTB7C as Susceptibility Genes to Ischemic Injury.
Du R, Zhou J, Lorenzano S, Liu W, Charoenvimolphan N, Qian B, Xu J, Wang J, Zhang X, Wang X, Berndt A, Devan WJ, Valant VJ, Wang J, Furie KL, Rosand J, Rost N, Friedlander RM, Paigen B, Weiss ST.
Stroke. 2015 Dec;46(12):3514-22. doi: 10.1161/STROKEAHA.115.010767. Epub 2015 Nov 5.
Role of Acute Lesion Topography in Initial Ischemic Stroke Severity and Long-Term Functional Outcomes.
Wu O, Cloonan L, Mocking SJ, Bouts MJ, Copen WA, Cougo-Pinto PT, Fitzpatrick K, Kanakis A, Schaefer PW, Rosand J, Furie KL, Rost NS.
Stroke. 2015 Sep;46(9):2438-44. doi: 10.1161/STROKEAHA.115.009643. Epub 2015 Jul 21.
COX-2 rs20417 Polymorphism Is Associated with Stroke and White Matter Disease.
Oliveira-Filho J, Ornellas AC, Zhang CR, Oliveira LM, Araújo-Santos T, Borges VM, Ventura LM, Reis FJ, Aras R, Fernandes AM, Rosand J, Greenberg SM, Furie KL, Rost NS.
J Stroke Cerebrovasc Dis. 2015 Aug;24(8):1817-22. doi:10.1016/j.jstrokecerebrovasdis.2015.04.018. Epub 2015 May 6.
Metabolic determinants of white matter hyperintensity burden in patients with ischemic stroke.
Cloonan L, Fitzpatrick KM, Kanakis AS, Furie KL, Rosand J, Rost NS.
Atherosclerosis. 2015 May;240(1):149-53. doi: 10.1016/j.atherosclerosis.2015.02.052. Epub 2015 Mar 2.
B-type natriuretic peptides help in cardioembolic stroke diagnosis: pooled data meta-analysis.
Llombart V, Antolin-Fontes A, Bustamante A, Giralt D, Rost NS, Furie K, Shibazaki K, Biteker M, Castillo J, Rodríguez-Yáñez M, Fonseca AC, Watanabe T, Purroy F, Zhixin W, Etgen T, Hosomi N, Jafarian Kerman SR, Sharma JC, Knauer C, Santamarina E, Giannakoulas G, García-Berrocoso T, Montaner J.
Stroke. 2015 May;46(5):1187-95. doi: 10.1161/STROKEAHA.114.008311. Epub 2015 Mar 12. Review.
Determinants of white matter hyperintensity burden differ at the extremes of ages of ischemic stroke onset.
Zhang CR, Cloonan L, Fitzpatrick KM, Kanakis AS, Ayres AM, Furie KL, Rosand J, Rost NS.
J Stroke Cerebrovasc Dis. 2015 Mar;24(3):649-54. doi: 10.1016/j.jstrokecerebrovasdis.2014.10.016. Epub 2014 Nov 6.
Segmentation of cerebrovascular pathologies in stroke patients with spatial and shape priors.
Dalca AV, Sridharan R, Cloonan L, Fitzpatrick KM, Kanakis A, Furie KL, Rosand J, Wu O, Sabuncu M, Rost NS, Golland P.
MICCAI. 2014;17(Pt 2):773-80.
White matter hyperintensity volume correlates with matrix metalloproteinase-2 in acute ischemic stroke.
Corbin ZA, Rost NS, Lorenzano S, Kernan WN, Parides MK, Blumberg JB, Milbury PE, Arai K, Hartdegen SN, Lo EH, Feske SK, Furie KL.
J Stroke Cerebrovasc Dis. 2014 Jul;23(6):1300-6. doi: 10.1016/j.jstrokecerebrovasdis.2013.11.002. Epub 2014 Jan 16.
17q25 Locus is associated with white matter hyperintensity volume in ischemic stroke, but not with lacunar stroke status.
Adib-Samii P, Rost N, Traylor M, Devan W, Biffi A, Lanfranconi S, Fitzpatrick K, Bevan S, Kanakis A, Valant V, Gschwendtner A, Malik R, Richie A, Gamble D, Segal H, Parati EA, Ciusani E, Holliday EG, Maguire J, Wardlaw J, Worrall B, Bis J, Wiggins KL, Longstreth W, Kittner SJ, Cheng YC, Mosley T, Falcone GJ, Furie KL, Leiva-Salinas C, Lau BC, Saleem Khan M; Australian Stroke Genetics Collaborative; Wellcome Trust Case-Control Consortium-2 (WTCCC2); METASTROKE, Sharma P, Fornage M, Mitchell BD, Psaty BM, Sudlow C, Levi C, Boncoraglio GB, Rothwell PM, Meschia J, Dichgans M, Rosand J, Markus HS; International Stroke Genetics Consortium.
Stroke. 2013 Jun;44(6):1609-15. doi: 10.1161/STROKEAHA.113.679936. Epub 2013 May 14.
Common variants within oxidative phosphorylation genes influence risk of ischemic stroke and intracerebral hemorrhage.
Anderson CD, Biffi A, Nalls MA, Devan WJ, Schwab K, Ayres AM, Valant V, Ross OA, Rost NS, Saxena R, Viswanathan A, Worrall BB, Brott TG, Goldstein JN, Brown D, Broderick JP, Norrving B, Greenberg SM, Silliman SL, Hansen BM, Tirschwell DL, Lindgren A, Slowik A, Schmidt R, Selim M, Roquer J, Montaner J, Singleton AB, Kidwell CS, Woo D, Furie KL, Meschia JF, Rosand J; International Stroke Genetics Consortium.
Stroke. 2013 Mar;44(3):612-9. doi: 10.1161/STROKEAHA.112.672089. Epub 2013 Jan 29.
Statin therapy and outcome after ischemic stroke: systematic review and meta-analysis of observational studies and randomized trials.
Ní Chróinín D, Asplund K, Åsberg S, Callaly E, Cuadrado-Godia E, Díez-Tejedor E, Di Napoli M, Engelter ST, Furie KL, Giannopoulos S, Gotto AM Jr, Hannon N, Jonsson F, Kapral MK, Martí-Fàbregas J, Martínez-Sánchez P, Milionis HJ, Montaner J, Muscari A, Pikija S, Probstfield J, Rost NS, Thrift AG, Vemmos K, Kelly PJ.
Stroke. 2013 Feb;44(2):448-56. doi: 10.1161/STROKEAHA.112.668277. Epub 2013 Jan 3. Review.
Quantification and Analysis of Large Multimodal Clinical Image Studies: Application to Stroke.
Sridharan R, Dalca AV, Fitzpatrick KM, Cloonan L, Kanakis A, Wu O, Furie KL, Rosand J, Rost NS, Golland P.
Multimodal Brain Image Anal (2013). 2013;8159:18-30. doi: 10.1007/978-3-319-02126-3_3.
Severity of leukoaraiosis in large vessel atherosclerotic disease.
Chutinet A, Biffi A, Kanakis A, Fitzpatrick KM, Furie KL, Rost NS.
AJNR Am J Neuroradiol. 2012 Sep;33(8):1591-5. doi: 10.3174/ajnr.A3015. Epub 2012 Mar 15.
Genome-wide association analysis of ischemic stroke in young adults.
Cheng YC, O'Connell JR, Cole JW, Stine OC, Dueker N, McArdle PF, Sparks MJ, Shen J, Laurie CC, Nelson S, Doheny KF, Ling H, Pugh EW, Brott TG, Brown RD Jr, Meschia JF, Nalls M, Rich SS, Worrall B, Anderson CD, Biffi A, Cortellini L, Furie KL, Rost NS, Rosand J, Manolio TA, Kittner SJ, Mitchell BD.
G3 (Bethesda). 2011 Nov;1(6):505-14. doi: 10.1534/g3.111.001164. Epub 2011 Nov 1.
Are myocardial infarction--associated single-nucleotide polymorphisms associated with ischemic stroke?
Cheng YC, Anderson CD, Bione S, Keene K, Maguire JM, Nalls M, Rasheed A, Zeginigg M, Attia J, Baker R, Barlera S, Biffi A, Bookman E, Brott TG, Brown RD Jr, Chen F, Chen WM, Ciusani E, Cole JW, Cortellini L, Danesh J, Doheny K, Ferrucci L, Grazia Franzosi M, Frossard P, Furie KL, Golledge J, Hankey GJ, Hernandez D, Holliday EG, Hsu FC, Jannes J, Kamal A, Khan MS, Kittner SJ, Koblar SA, Lewis M, Lincz L, Lisa A, Matarin M, Moscato P, Mychaleckyj JC, Parati EA, Parolo S, Pugh E, Rost NS, Schallert M, Schmidt H, Scott RJ, Sturm JW, Yadav S, Zaidi M, Boncoraglio GB, Levi CR, Meschia JF, Rosand J, Sale M, Saleheen D, Schmidt R, Sharma P, Worrall B, Mitchell BD; GARNET Collaborative Research Group; GENEVA Consortium; International Stroke Genetics Consortium.
Stroke. 2012 Apr;43(4):980-6. doi: 10.1161/STROKEAHA.111.632075. Epub 2012 Feb 23.
Erratum in:Stroke. 2015 Aug;46(8):e204.
Brain natriuretic peptide predicts functional outcome in ischemic stroke.
Rost NS, Biffi A, Cloonan L, Chorba J, Kelly P, Greer D, Ellinor P, Furie KL.
Stroke. 2012 Feb;43(2):441-5. doi: 10.1161/STROKEAHA.111.629212. Epub 2011 Nov 23.
Severity of leukoaraiosis determines clinical phenotype after brain infarction.
Arsava EM, Bayrlee A, Vangel M, Rost NS, Rosand J, Furie KL, Sorensen AG, Ay H.
Neurology. 2011 Jul 5;77(1):55-61. doi: 10.1212/WNL.0b013e318221ad02. Epub 2011 Jun 22.
Statin treatment and functional outcome after ischemic stroke: case-control and meta-analysis.
Biffi A, Devan WJ, Anderson CD, Cortellini L, Furie KL, Rosand J, Rost NS.
Stroke. 2011 May;42(5):1314-9. doi: 10.1161/STROKEAHA.110.605923. Epub 2011 Mar 17. Review.
White matter hyperintensity volume is increased in small vessel stroke subtypes.
Rost NS, Rahman RM, Biffi A, Smith EE, Kanakis A, Fitzpatrick K, Lima F, Worrall BB, Meschia JF, Brown RD Jr, Brott TG, Sorensen AG, Greenberg SM, Furie KL, Rosand J.
Neurology. 2010 Nov 9;75(19):1670-7. doi: 10.1212/WNL.0b013e3181fc279a.
White matter hyperintensity burden and susceptibility to cerebral ischemia.
Rost NS, Fitzpatrick K, Biffi A, Kanakis A, Devan W, Anderson CD, Cortellini L, Furie KL, Rosand J.
Stroke. 2010 Dec;41(12):2807-11. doi: 10.1161/STROKEAHA.110.595355. Epub 2010 Oct 14.
Common mitochondrial sequence variants in ischemic stroke.
Anderson CD, Biffi A, Rahman R, Ross OA, Jagiella JM, Kissela B, Cole JW, Cortellini L, Rost NS, Cheng YC, Greenberg SM, de Bakker PI, Brown RD Jr, Brott TG, Mitchell BD, Broderick JP, Worrall BB, Furie KL, Kittner SJ, Woo D, Slowik A, Meschia JF, Saxena R, Rosand J; International Stroke Genetics Consortium.
Ann Neurol. 2011 Mar;69(3):471-80. doi: 10.1002/ana.22108. Epub 2010 Sep 13.
Principal-component analysis for assessment of population stratification in mitochondrial medical genetics.
Biffi A, Anderson CD, Nalls MA, Rahman R, Sonni A, Cortellini L, Rost NS, Matarin M, Hernandez DG, Plourde A, de Bakker PI, Ross OA, Greenberg SM, Furie KL, Meschia JF, Singleton AB, Saxena R, Rosand J.
Am J Hum Genet. 2010 Jun 11;86(6):904-17. doi: 10.1016/j.ajhg.2010.05.005. Epub 2010 May 27.
Determinants of white matter hyperintensity volume in patients with acute ischemic stroke.
Rost NS, Rahman R, Sonni S, Kanakis A, Butler C, Massasa E, Cloonan L, Gilson A, Delgado P, Chang Y, Biffi A, Jimenez-Conde J, Besanger A, Silva G, Smith EE, Rosand J, Furie KL.
J Stroke Cerebrovasc Dis. 2010 May;19(3):230-5. doi:10.1016/j.jstrokecerebrovasdis.2009.05.007.
Failure to validate association between 12p13 variants and ischemic stroke.
International Stroke Genetics Consortium; Wellcome Trust Case-Control Consortium 2.
N Engl J Med. 2010 Apr 22;362(16):1547-50. doi: 10.1056/NEJMc0910050. No abstract available.
Chromosome 9p21 in ischemic stroke: population structure and meta-analysis.
Anderson CD, Biffi A, Rost NS, Cortellini L, Furie KL, Rosand J.
Stroke. 2010 Jun;41(6):1123-31. doi: 10.1161/STROKEAHA.110.580589. Epub 2010 Apr 15.
Hyperlipidemia and reduced white matter hyperintensity volume in patients with ischemic stroke.
Jimenez-Conde J, Biffi A, Rahman R, Kanakis A, Butler C, Sonni S, Massasa E, Cloonan L, Gilson A, Capozzo K, Cortellini L, Ois A, Cuadrado-Godia E, Rodriguez-Campello A, Furie KL, Roquer J, Rosand J, Rost NS.
Stroke. 2010 Mar;41(3):437-42. doi: 10.1161/STROKEAHA.109.563502. Epub 2010 Feb 4.
Erratum in: Stroke. 2017 Aug;48(8):e241.
Remote supervision of IV-tPA for acute ischemic stroke by telemedicine or telephone before transfer to a regional stroke center is feasible and safe.
Pervez MA, Silva G, Masrur S, Betensky RA, Furie KL, Hidalgo R, Lima F, Rosenthal ES, Rost N, Viswanathan A, Schwamm LH.
Stroke. 2010 Jan;41(1):e18-24. doi: 10.1161/STROKEAHA.109.560169. Epub 2009 Nov 12.
Severity of leukoaraiosis correlates with clinical outcome after ischemic stroke.
Arsava EM, Rahman R, Rosand J, Lu J, Smith EE, Rost NS, Singhal AB, Lev MH, Furie KL, Koroshetz WJ, Sorensen AG, Ay H.
Neurology. 2009 Apr 21;72(16):1403-10. doi: 10.1212/WNL.0b013e3181a18823.